Are the mRNA COVID vaccines safe for me?
By now, you might have heard about it from a frontline worker or a friend who has received the COVID-19 vaccine. As with other established vaccines, in rare instances, a person who receives the vaccine may experience severe allergic reactions. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose of the COVID-19 Vaccine.
What are some possible side effects of the vaccine? How do I manage them?
The occurrence of side effects signifies that the vaccine is successfully invoking an immune response by our body. Most side effects are mild or moderate and usually go away within a few days. Hence, vaccine recipients should complete the second dose in order to have optimal antibody response.Post-vaccination symptoms from both doses observed in the Phase 3 study of the Pfizer-BioNTech vaccine include:
- Pain at the injection site (84%)
- Fatigue (63%)
- Headache (55%)
- Muscle pain (38%)
- Chill (32%)
- Joint pain (24%)
- Fever (14%)
- Injection site swelling/redness (10%)
- Lymphadenopathy (0.3%)
The table below lists some common side effects that have been reported with this vaccine, and how to manage them.
| Side effects | How to manage them |
|---|---|
| Pain, redness and swelling at the injection site | Paracetamol 1 to 2 tablets every 6 hours as needed |
| Fever, chills | |
| Headache, muscle pain , joint pain | |
| Tiredness | Rest |
| Lymph node swelling in the neck or arms | Usually gets better by itself within a week |
See a doctor if:
- The side effects persist or get worse
- The fever persists for more than 48 hours (2 days)
How will I know if I am having a severe reaction to the vaccine?
After a nurse administers the vaccine, recipients will be led to a waiting area to rest for 30 minutes. Medical staff are present and ready to check on your health condition so that any severe allergic reactions such as anaphylaxis can be detected and treated promptly.
What is anaphylaxis?
Anaphylaxis is a sudden, severe and life-threatening reaction that can occur within minutes to hours from exposure to an allergen.
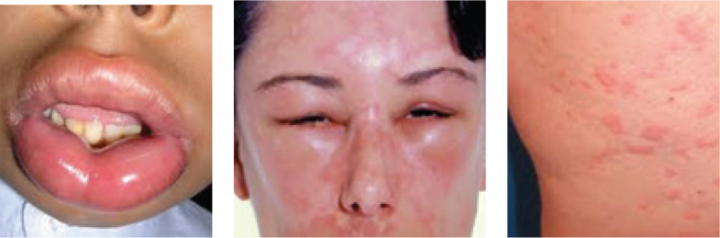
The symptoms include:
- Skin reactions such as hives and flushing
- Swelling of the lips, tongue, face or throat, which can cause wheezing and trouble breathing
- Feeling light-headed or collapsing due to low blood pressure
- Confusion
- Nausea
- Vomiting
- Diarrhoea
- Abdominal cramps
If you experience any of the above symptoms, seek immediate medical attention from our medical staff. Call 995 or go to the nearest A&E immediately.
Which groups are at risk of anaphylaxis and should not be vaccinated?
Do not get vaccinated if you:
- Have a history of having an allergic reaction to a previous dose of the Pfizer-BioNTech COVID-19 Vaccine or any of its components.
- Have a history of severe drug reactions or anaphylaxis to other drugs, vaccines, food, insect stings, or unknown triggers.
- Have a history of having been prescribed an Epi-Pen.
Vaccination can proceed in:
- Persons with a family history (but NOT a personal history) of anaphylaxis.
- Persons with atopy such as eczema, allergic rhinitis or well-controlled asthma.
- Persons with multiple non-severe drug allergies.
Will patients with immunocompromised conditions or treatments benefit from a COVID-19 vaccination?
Immunocompromised persons are likely to be at an increased risk of developing severe COVID-19 symptoms if infected. Based on available data, they do not appear to be at an increased risk of adverse effects from COVID-19 vaccination. Therefore, although vaccination is likely to be less effective in immunocompromised persons and some protection will likely be engendered. Persons who are on lower amounts of immunosuppression can continue to be vaccinated. For those on short-term immunosuppression, it is advisable to temporarily postpone vaccination to a later date. Until more data becomes available, this vaccine is not recommended for severely immunosuppressed persons, such as those with active cancer, are on cancer treatment, are on other immunotherapy for non-cancer conditions, or have a HIV infection with CD4 < 200.
Please speak to your doctor if you would like to find out more information about this.
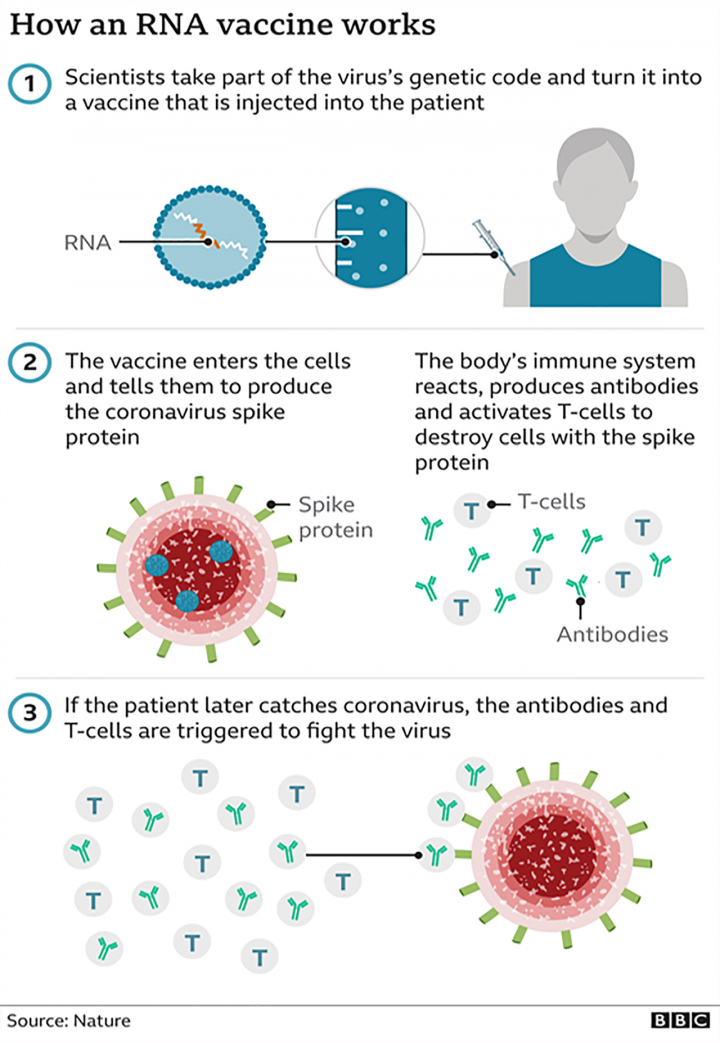
Can the vaccine cause a recipient to develop a COVID-19 infection? Will the vaccine alter our genetic make-up?
Is the COVID-19 vaccination safe for those with chronic illnesses such as hypertension, high cholesterol, heart disease and diabetes?
The study population for Pfizer-BioNTech’s phase 3 trials included persons with chronic diseases and there were no safety concerns reported in this group. Persons with chronic illnesses are hence recommended to receive the vaccine for their protection as well as protecting their loved ones. Those on anti-platelet agents (eg. aspirin), anti-coagulation (eg. warfarin) can also be vaccinated. However, five minutes of firm pressure must be applied to injection site to prevent bruising.
There had been reports of deaths in frail elderly persons following vaccination with the Pfizer-BioNTech COVID-19 vaccine in other countries. Can a frail elderly person still receive the vaccination?
The Singapore Health Sciences Authority (HSA) has been assessing the implications of the reported deaths. HSA is in touch with overseas regulatory authorities for further information. There is currently no evidence of a direct link between the elderly deaths and the vaccine. There were also no safety concerns reported from the UK, where more than 1 million elderly persons above the age of 80, including nursing home residents, have been vaccinated. It is recommended that the elderly continue to be vaccinated due to the higher risk of serious, life-threatening disease and death from COVID-19 infection.
Please speak to your doctor should you have any concerns.
How soon after vaccination can a woman try to conceive? Can a woman be vaccinated if she is pregnant? If she received the first dose of vaccine and then became pregnant, can she get the second dose?
There is currently no data on the safety of COVID-19 vaccines during pregnancy.
Women who are planning a pregnancy are advised to consider deferring conception for one month after completing the second dose. This does not apply to male vaccine recipients. Women who become pregnant after the first dose and before the second dose should not receive the second dose of vaccine, but should postpone it until after delivery
Can a woman who is breastfeeding receive the COVID-19 vaccine?
Women who are breastfeeding can be vaccinated. However, out of caution, women can consider suspending breastfeeding for five to seven days after receiving the vaccine. If a woman feels she is unable to suspend breastfeeding and still wishes to get vaccinated, she can do so.
Be the First to Know!
Like what you see? Receive more health and wellness articles from Raffles Medical Group to aid you in leading a healthy lifestyle.


